Facility Services for Life Sciences
Protecting Your Right To Operate
Setting the Standard
Driven by Distinction
At SBM, we support life sciences facilities that operate in an environment where precision, safety, and compliance are required. Your success and right to operate hinges on maintaining stringent standards that not only protect the integrity of the work but also ensure the safety of your employees and the public. Even a minor lapse in facility management can lead to significant risks, including regulatory violations, compromised research, production delays, or threats to public health.






Comprehensive Soft Service Offerings
SBM offers a full spectrum of soft services to support your daily operations and maintain a healthy environment for your staff and visitors:

Precision in Motion
Specialized Life Sciences Facility Services
To address the specific needs of life sciences facilities, SBM provides services that ensure operational compliance, support inspection readiness, and right to operate:
GMP (Good Manufacturing Practices)
Lab Services
Glass Wash
Gowning Service
Aseptic/Fill Line Sanitizing
Hazardous Material Management
Clean Build Fab Construction Support
SME Consulting for Inspection Readiness
Consumable Inventory Management
GMP Environment Training
Our associates are trained specifically in GMP environments, with emphasis on supplementing and supporting our client’s right to operate policy and training. This is vital for our service technicians to develop expertise in the culture of compliance, highlights the importance of their work, and instills pride in the work they do.
Precaution in Action
A Commitment to Compliance
Our culture of compliance is integral to our operations, with core processes designed to deliver consistent, high-quality services that meet regulatory standards. Our training programs, quality audits, Root Cause Analysis (RCA), and agency inspection readiness support ensure your facility remains compliant and operational.
Additionally, SBM managers conduct GMP Associate Audits, observing and evaluating team members based on criteria established from client SOPs and requirements. These audits assess employee knowledge, technical proficiency, safety practices, chemical solutions, equipment preparation and GDP and log sheet compliance. They also proactively detect additional training needs before any situations develop into compliance or quality concerns.
OSHA VPP
Star Status
Americas Safest Companies
by EHS Today
Governor’s Occupational Safety & Health Awards
National Safety Council
Occupational Excellence
Understanding GMP Spaces
Our Systems & Processes
SBM proudly supports some of the world’s leading life sciences facilities. Our focus on quality, safety, and compliance ensures we meet the high standards required in this industry. Our teams’ dedication and supplemental processes have evolved over the 32 years we have provided GMP facility services, resulting in ZERO regulatory agency observations attributed to SBM service delivery.
Our Controlled Environments (CE) cleaning service team is dedicated to ensuring our clients' inspection readiness and maintaining their right to operate. This specialized team works as an extension of your quality department, providing expert knowledge in cleaning and sanitization, conducting site assessments, assisting with regulatory inspections, conducting Root Cause Analysis (RCA) and consulting Standard Operating Procedures (SOPs).
Client Support
Support client's Right to Operate through policies and targeted training.
Conducting Gemba Walks
We conduct Gemba walks to observe staff, verify SOP compliance, evaluate performance, and proactively identify training needs.
Corrective Action
We identify, correct, and track error types, causes, and corrective actions in our enterprise software platform.
Facility Inspections
We perform facility inspections to measure service results and develop follow-up actions for gaps.
Regulatory Inspections
We are committed to ensuring client facilities pass all regulatory inspections with zero sanitizing program observations.
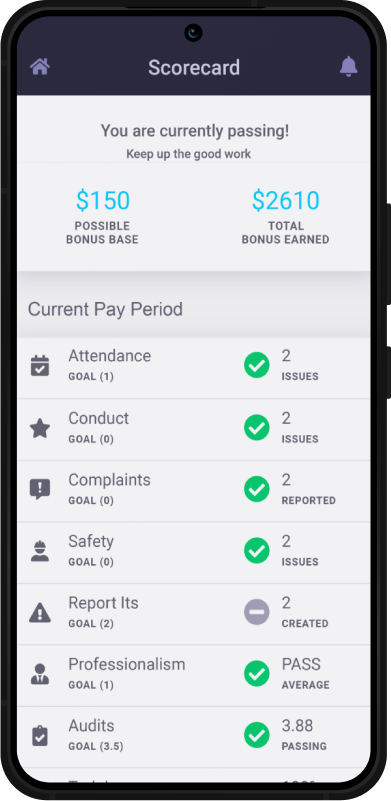
Program Performance
We create and track client-aligned KPIs, goals, and results captured monthly to measure overall program performance.
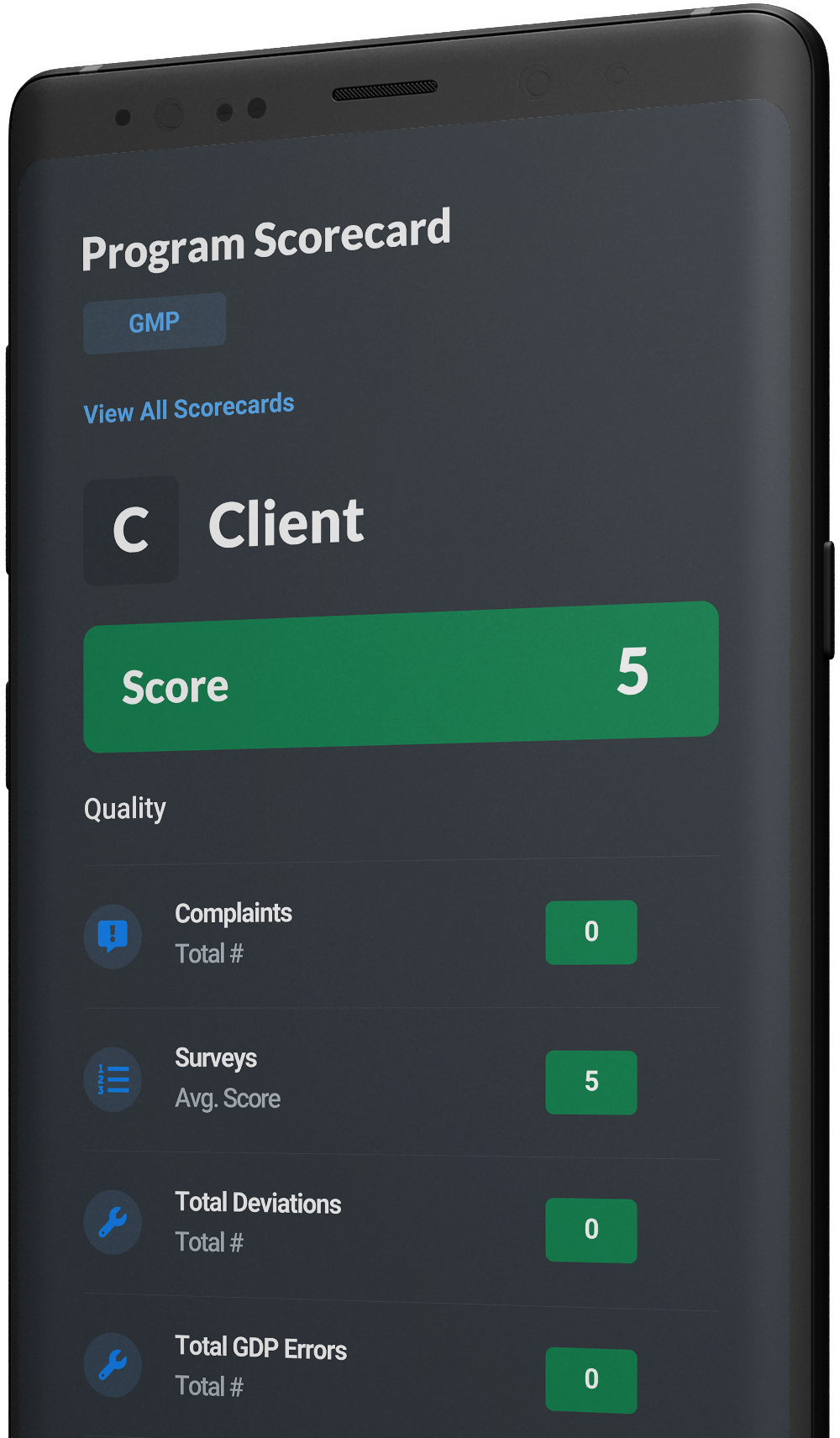
GMP Site Scorecards
Site scorecards provide transparent monthly results that foster valuable conversations with clients. With over 40 KPIs to choose from, site scorecards are uniquely designed for each account to align with what our client finds most important and help establish goals or control limits and then manage our performance against mutually agreed upon targets.
GMP-speicifc KPIs include:
Deviations
GDP Errors
SOP Violations
GMP Associate Audits
Client Training Compliance
Periodical Completions
Impacts to Production

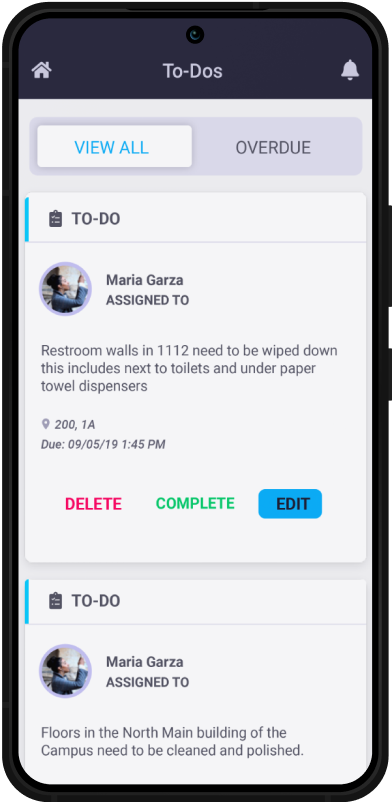
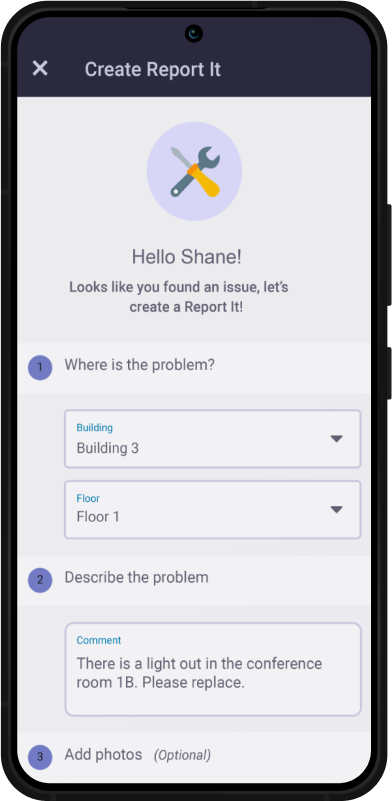
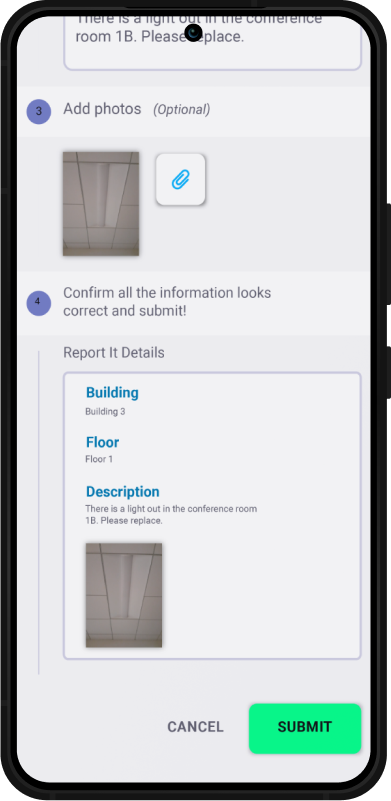
Why Choose SBM?
Our Platform Technology
Always Makes the
Connection
Your facilities are getting smarter, and the demands of building occupants continue to evolve. You need a service partner who can keep up.
Choosing SBM means partnering with a company that understands the critical importance of maintaining a compliant and efficient facility. Our innovative solutions, extensive industry experience, and commitment to excellence make us the ideal partner for your facility needs.
Contact us today to discover how SBM can support your life sciences facility.
